Different production processes lead to significant differences in the structure of magnesia-chrome materials, which in turn affect the performance of magnesia-chrome bricks. For example, directly bonded magnesia-chrome bricks have good thermal shock resistance, while fused-rebonded magnesia-chrome bricks have strong corrosion resistance. The performance of semi-rebonded magnesia-chrome bricks lies somewhere in between. To this end, this article will discuss in detail the impact of production processes on the performance of magnesia-chrome bricks.

Impact on Microstructure and Properties
Figures 3-5 show the apparent porosity and bulk density of magnesia-chromium refractories produced using different production processes.
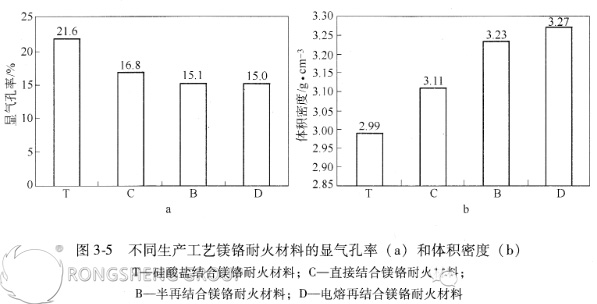
Figures 3-5 show significant differences in porosity and bulk density among magnesia-chrome refractories produced using different manufacturing processes. The porosity trend follows the following pattern: silicate-bonded magnesia-chrome refractories (T) > directly-bonded magnesia-chrome refractories (C) > semi-rebonded magnesia-chrome refractories (B) > fused-bonded magnesia-chrome refractories (D). The bulk density shows the opposite trend.
This is primarily due to differences in manufacturing processes. High-quality magnesia-chrome bricks (fused-bonded, semi-rebonded, and directly-bonded) are produced using high-purity raw materials, high molding pressures, and high firing temperatures. Consequently, the resulting products have a dense structure, low porosity, and high bulk density. This demonstrates that the production process significantly influences refractory performance.
Impact on Hot Strength
The impact of the production process on the high-temperature strength of magnesia-chromium refractory materials is shown in Figures 3-6.
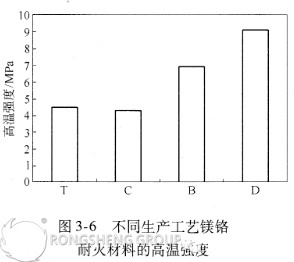
Figures 3-6 show that the high-temperature strength of magnesia-chrome refractories produced by different processes varies as follows: silicate-bonded magnesia-chrome refractories < directly bonded magnesia-chrome refractories < semi-rebonded magnesia-chrome refractories < fused-bonded magnesia-chrome refractories. Since high-temperature flexural strength primarily reflects the bonding of minerals within the brick, fused-bonded magnesia-chrome bricks possess the highest bonding strength, and therefore offer the best resistance to erosion and erosion at high temperatures.
Due to the different liquid phases, the permeability between grains is lower than that between identical grains. When the liquid phase content remains constant, the presence of a second solid phase increases solid-solid contact, thereby improving the brick’s high-temperature strength. When producing fused-bonded or semi-rebonded magnesia-chrome bricks, the addition of presynthesized magnesia-chrome sand increases the second solid phase content, enhancing the brick’s high-temperature strength.
Impact on Thermal Shock Stability
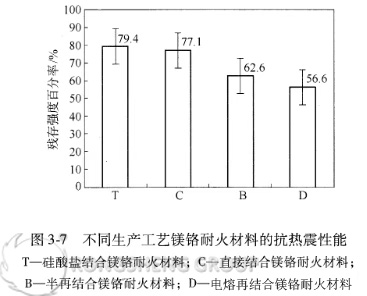
The thermal shock stability of various magnesia-chrome bricks was compared using the percentage of residual strength after a single air-cooling cycle at 1100°C. The results are shown in Figure 3-7. As shown in Figure 3-7, among the magnesia-chrome bricks produced using different production processes, conventional magnesia-chrome bricks have the best thermal shock stability, followed by semi-rebonded magnesia-chrome bricks and directly bonded magnesia-chrome bricks, and fused-rebonded magnesia-chrome bricks have the worst.
Effect on Slag Resistance
Since intergranular spinel precipitates from the silicate liquid phase, the presence of a small amount of SiO2 favors its formation. However, an increased SiO2 content significantly increases the liquid phase volume, reducing the high-temperature strength of the magnesia-chrome brick and facilitating the infiltration of foreign melts along the liquid phase channels, exacerbating corrosion. A high CaO content significantly lowers the temperature at which the liquid phase appears, adversely affecting high-temperature strength and corrosion resistance. This is particularly true when the Al2O3 and Fe2O3 contents in the magnesia-chrome brick are high. Therefore, when producing high-quality magnesia-chrome bricks, chromium ores with low SiO2, CaO, and Fe2O3 (FeO) contents, and high Cr2O3 and Al2O3 contents, should be used as raw materials.
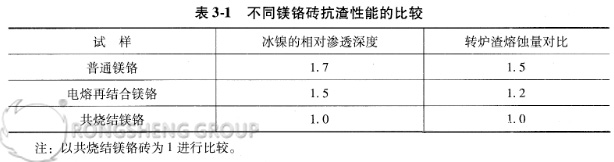
Tests were conducted on the corrosion resistance of low-grade nickel matte and converter slag using conventional magnesia-chrome bricks (i.e., silicate-bonded magnesia-chrome bricks), fused-bonded magnesia-chrome bricks, and co-sintered magnesia-chrome bricks currently used in China. The results are shown in Table 3-1.
Effects of Chromium Ore on the Performance of Magnesia-Chrome Bricks
Chromium ore is the primary raw material for the production of magnesia-chrome bricks. Its primary impurity content varies depending on its source. Furthermore, the amount of chromium ore added also affects the performance of magnesia-chrome bricks. This section will primarily explain the effects of chromium ore source and addition on the performance of directly bonded magnesia-chrome bricks.

Effect of Chromium Ore Particle Size
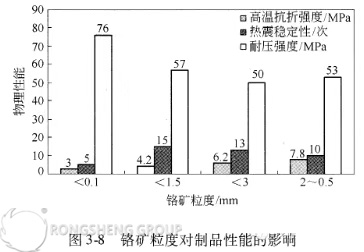
While domestic high-chrome ore (Cr2O3 > 53%) contains significantly less iron oxide than South African chrome ore, it does have a higher silica content. Therefore, we investigated the effect of the particle size composition of domestic high-chrome ore on the performance of directly bonded magnesia-chrome bricks. The results are shown in Figure 3-8. As shown in Figure 3-8, the compressive strength of the products increases with decreasing critical chrome ore particle size, while the high-temperature flexural strength (1400°C, 0.5h) decreases with decreasing critical chrome ore particle size. Thermal shock resistance peaks at a critical chrome ore particle size of 1.5mm.
Effect of Chromium Ore Addition
Directly bonded magnesia-chromium samples with varying Cr₂O₃ contents were prepared by mixing fused magnesia and South African chromium ore in varying ratios. These samples were fired in a high-temperature kiln at 1740°C. The chemical compositions of the fused magnesia, South African chromium ore, and magnesia-chromium samples are shown in Table 3-2.
The effects of South African chromium ore on the performance of directly bonded magnesia-chrome bricks are shown in Figures 3-9 to 3-11.
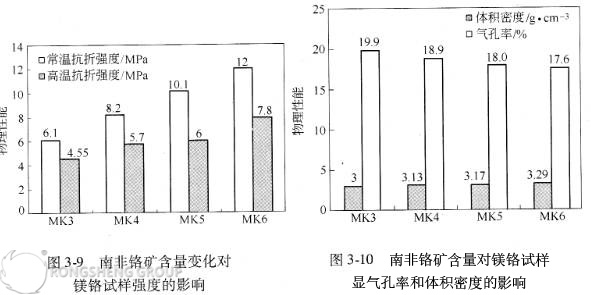
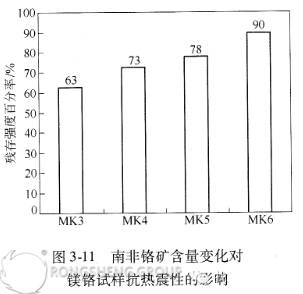
Figures 3-10 and 3-11 show that with increasing chromium ore content, the apparent porosity of the directly bonded magnesium-chromium samples decreases, the bulk density increases, and the thermal shock stability improves. Optical microscopy, SEM, and EDAX analysis indicate that after high-temperature calcination, the vast majority of the iron oxide in the South African chromium ore enters the periclase phase, forming a (MgO, FeO) solid solution or magnesium ferrite secondary spinel. As mentioned previously, the volume shrinks by 20% during the formation of (Mg, Fe)O. Furthermore, in the presence of CaO, a liquid phase appears at 1500°C, promoting dense sintering. This is one reason why the South African ore increases the bulk density and decreases the porosity of the sintered composite.
EDAX analysis of the magnesium fulvite phase composition reveals that the order of solid solution of the sesquioxides in periclase is: Fe₂O₃ > Cr₂O₃ > Al₂O₃. Although a certain amount of magnesium oxide diffuses into the ferrochrome spinel, the amount of sesquioxides (especially Fe₂O₃) from the ferrochrome spinel that enters the periclase is relatively greater than the amount of magnesium oxide that enters the ferrochrome composite spinel. Therefore, after high-temperature calcination, the composition of both the magnesia and the chrome ore changes, with a larger amount of secondary spinel forming in the periclase. Its typical composition (EDAX analysis, mass fraction) is: MgO 75.90%, Al₂O₃ 3.21%, Fe₂O₃ 15.49%, and Cr₂O₃ 5.42%. The composition of the chrome ore particles also varies significantly. The typical chemical composition of chrome ore particles (at the direct bonding boundary) is: MgO 21.69%, Cr₂O₃ 47.35%, Al₂O₃ 12.31%, and Fe₂O₃ 12.73%. Therefore, the degree of direct bonding in this directly bonded magnesia-chromium material is high. Consequently, increasing the chrome ore content significantly improves the physical properties of the magnesia-chromium sample.
The relationship between the corrosion resistance of directly bonded magnesia-chrome bricks to non-ferrous smelting media and the chromium ore content is shown in Figure 3-12.
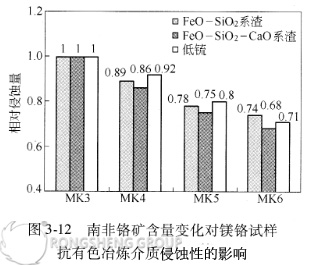
With the increase of Cr2O3 content in the sample, its corrosion resistance to non-ferrous smelting media increases significantly, as does its corrosion and penetration resistance.







